Breaking down the financial investment in developing a vaccine: the high cost of creating life-saving medicine
Vaccines are one of the most important medical advancements in history. They have saved millions of lives, eradicated devastating diseases, and drastically improved public health. But creating a vaccine is no easy task - it requires years of research, development, clinical trials and production. So how much does it cost to make a vaccine?
In this article, we will cover a quick recap on the types of vaccines, how they are developed and finally at a at the various costs associated with making them. Finally, we'll explore some potential strategies that could help reduce the overall cost of producing vaccines in order to make them more accessible around the world.
How does a vaccine work?
We'll see soon that there are different types of vaccines, but in general , the vaccine works by introducing a weakened or inactivated form of a virus or bacteria into your body. Your immune system then recognizes the invader and begins to fight it off, producing antibodies that help protect you from future infections. If you are then exposed to the germ in the future, your immune system can quickly destroy it before you become unwell. This is why once you get vaccinated for a certain disease, you are generally protected from getting it again. Our immune systems are designed to remember, and once exposed to one or more doses of vaccine, we typically remain protected against a disease for years, decades, or even a lifetime.
Types of vaccines
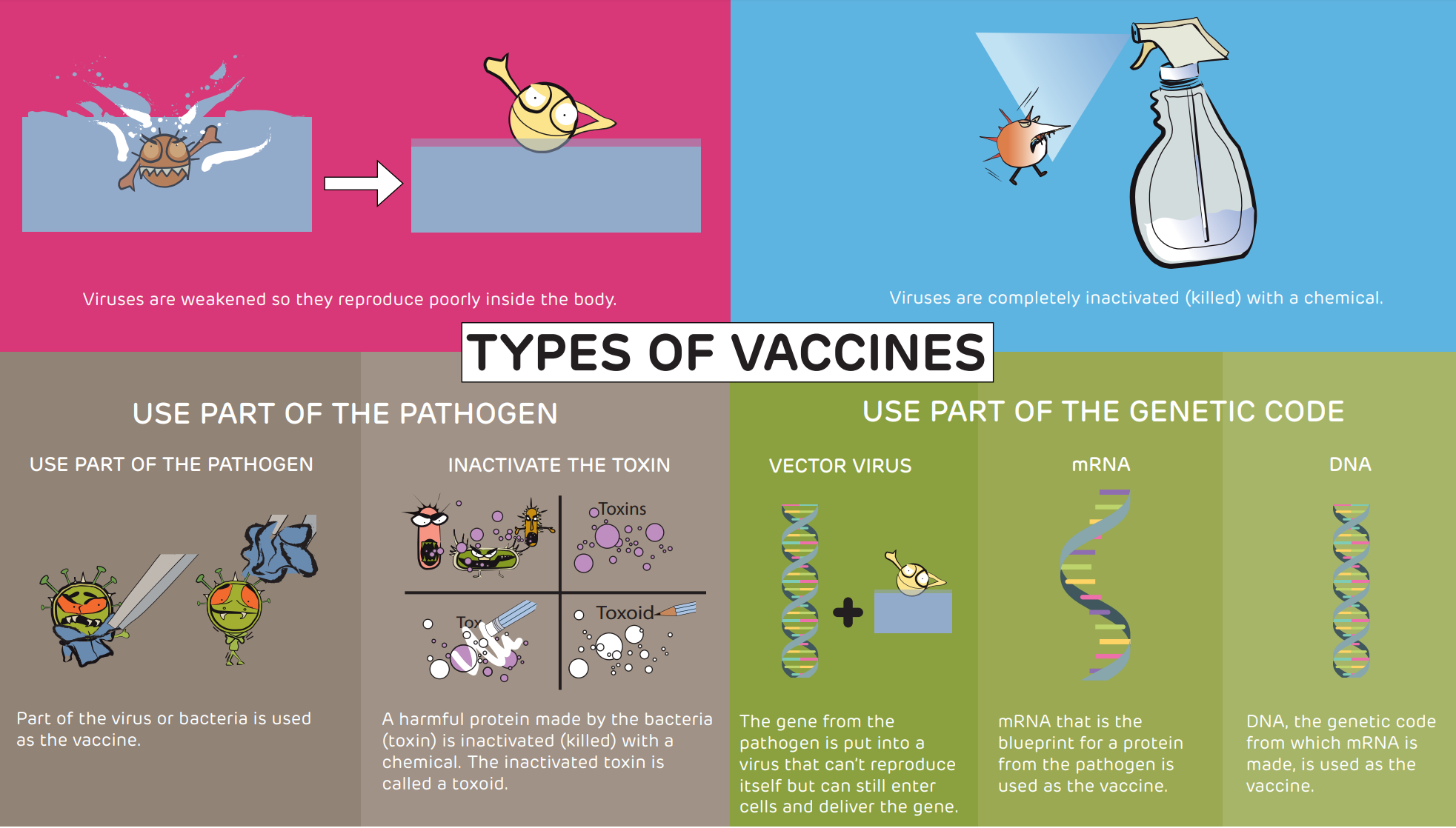
The Children Hospital of Philadelphia gives a good overview of the different types of vaccine and the processes needed to create them. I summarized the main techniques below:
- Weaken the virus: viruses are weakened so they reproduce very poorly once inside the body. The vaccines for measles, mumps, German measles (rubella), rotavirus and chickenpox (varicella) vaccines are made this way. Because vaccine viruses don't reproduce very much, they don't cause disease, but vaccine viruses replicate well enough to induce "memory B cells" that protect against infection in the future.
- Inactivate the virus: viruses are completely inactivated (or killed) with a chemical. By killing the virus, it cannot possibly reproduce itself or cause disease. The inactivated polio, hepatitis A and influenza (shot) vaccines are made this way. Because the virus is still "seen" by the body, cells of the immune system that protect against disease are generated.
- Use part of the virus: just one part of the virus is removed and used as a vaccine. The hepatitis B and human papillomavirus (HPV) vaccines are made this way.
- Use part of the bacteria: some bacteria cause disease by making a harmful protein called a toxin. Several vaccines are made by taking toxins and inactivating them with a chemical. The diphtheria, tetanus and pertussis vaccines are made this way.
- Provide the genetic code (DNA, mRNA, or vectored viruses) for part of the virus: Using this strategy, the person who is vaccinated makes part of the virus to trigger the immune response. Some of the vaccines for COVID-19 are made this way.
How are vaccines developed
Developing a vaccine is an incredibly complex and expensive process that involves years of research, development, clinical trials and production. This process starts with researching the virus or disease in question to identify its specific characteristics so that an effective vaccine can be designed. Once a vaccine has been designed, it must go through rigorous testing before being approved for use. Clinical trials involve administering the vaccine to large groups of people in order to gauge its safety and effectiveness while also monitoring any potential side effects. After this phase is complete, regulatory bodies such as the FDA must approve it before it can be manufactured and distributed on a larger scale.
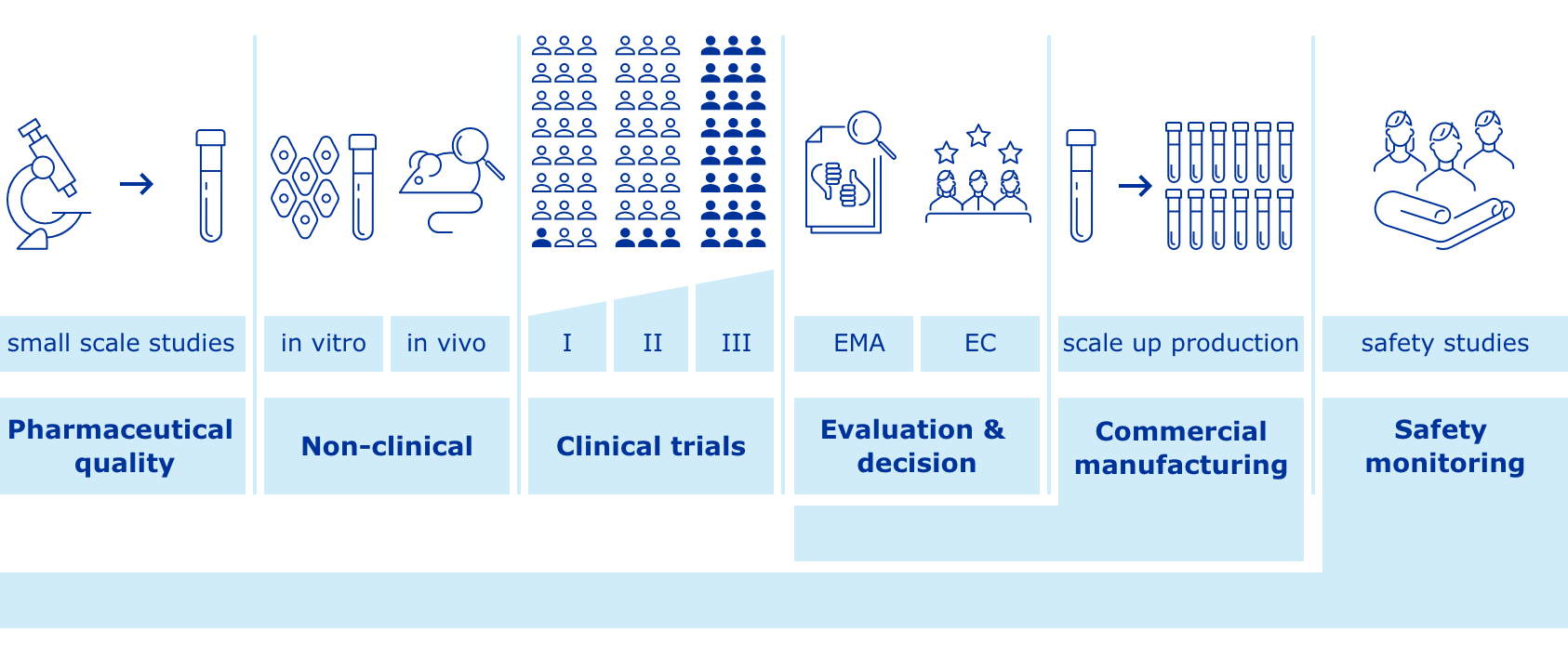
The general stages of the development cycle of a vaccine are:
- Exploratory stage: laboratory research is conducted for 2 to 5 years to identify antigens to include in a vaccine
- Pre-clinical stage: researchers conduct testing to assess vaccine candidates’ for their ability to elicit the desired immune response. Other areas of focus include short-term toxicology, formulation, and development of a scalable, efficient, and reproducible manufacturing process (2 years).
- Clinical development:
- Phase I: small groups of young, healthy adults receive the trial vaccine to confirm it generates an immune response, and determine the right dosage (2 years)
- Phase II: the vaccine is given to several hundred volunteers who have characteristics (such as age and physical health) similar to those for whom the new vaccine is intended (2 to 3 years)
- Phase III: the vaccine is given to thousands of people and tested for efficacy and safety (5 to 10 years). Many vaccines undergo Phase IV formal, ongoing studies after the vaccine is approved and licensed.
- Regulatory review and approval: if the candidate vaccine is determined to be safe and effective, an application is submitted to the vaccine authority (e.g. FDA in US, EMA in Europe), which may conduct its own testing. (up to 2 years).
- Manufacturing: scales up production of large quantities of the vaccine, ensuring all product meets the necessary regulatory requirements. On average, it takes between 12-36 months before distribution.
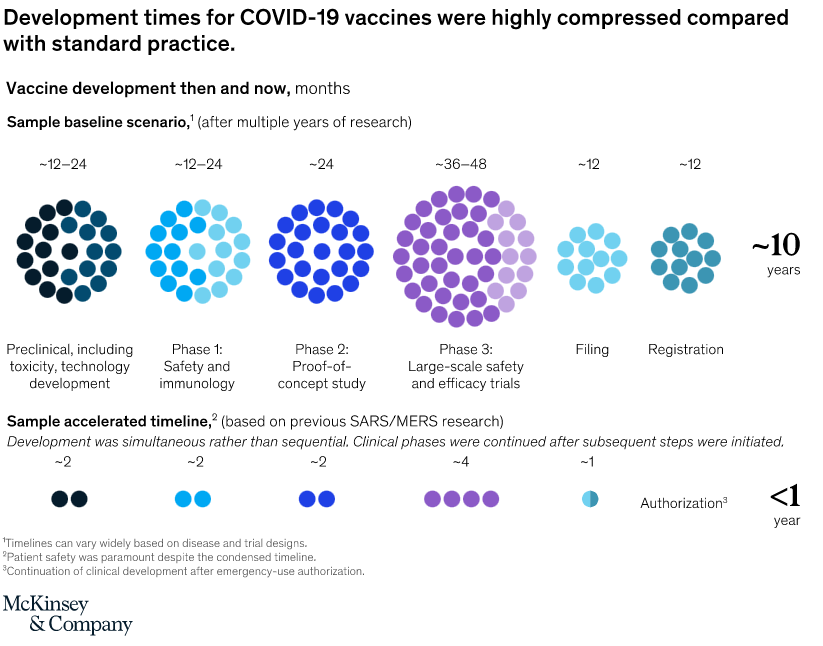
Why it was quick to create the COVID-19 vaccines?
When scientists began seeking a vaccine for the SARS-CoV-2 coronavirus in early 2020, they were careful not to promise quick success. The fastest any vaccine had previously been developed, from viral sampling to approval, was four years, for mumps in the 1960s.
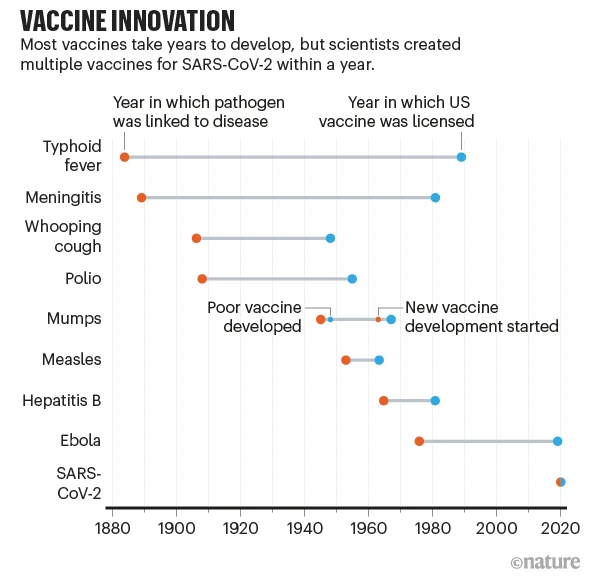
The world was able to develop COVID-19 vaccines so quickly because of years of previous research on related viruses and faster ways to manufacture vaccines, enormous funding that allowed firms to run multiple trials in parallel, and regulators moving more quickly than normal. Some of those factors might translate to other vaccine efforts, particularly speedier manufacturing platforms.
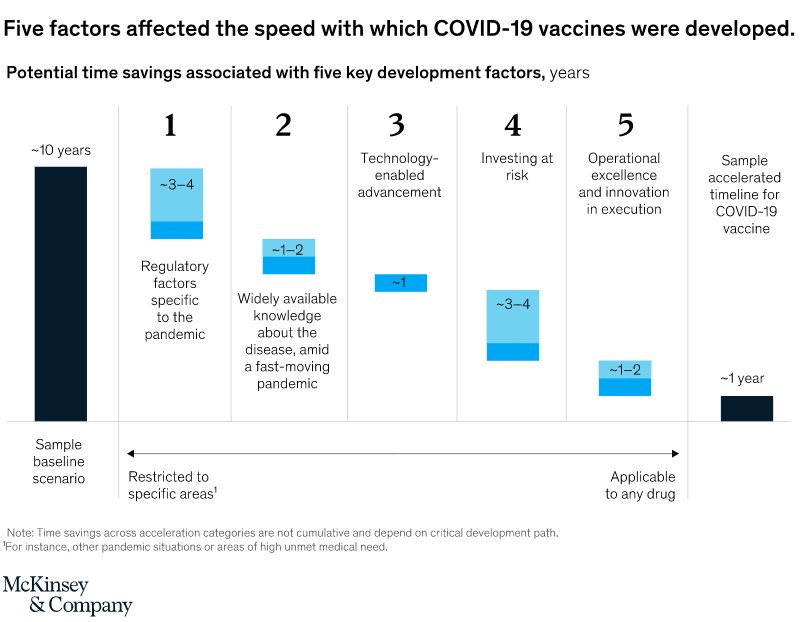
But there’s no guarantee. To repeat such rapid success will require similar massive funding for development, which is likely to come only if there is a comparable sense of social and political urgency. It will depend, too, on the nature of the pathogen. With SARS-CoV-2, a virus that mutates relatively slowly and that happens to belong to a well-studied family, scientists might — strange as it sounds — have got lucky.
The high cost of vaccine development
Analyzing the cost of developing a vaccine is not easy as there are many factors involved. In general, vaccine development from discovery to licensure can cost billions of dollars and has an average 94% chance of failure. This means that many (expensive) attempts have to be done before the successful vaccine can be distributed and monetized.
A team of researchers attempted to analyze the cost of developing a vaccine in a given portfolio of 11 epidemic infectious diseases. The total cost of progressing one epidemic infectious disease vaccine successfully through to end of phase 3 is dependent on the probability of success and on the shape of the vaccine research and development pipeline.
As the figure demonstrates, accounting for probability of success and assuming no clinical vaccine candidates exist for a given epidemic infectious disease, 11 to 21 preclinical candidates would be required if at least one of these were to progress through to end of phase 2, at a cost of $319–469 million ($137 million–$1.1 billion range).
Similarly, six to ten phase 1 candidates would be needed for at least one candidate to advance through to end of phase 2a, at a cost of $167–201 million ($61 million–$485 million range). Assuming vaccine candidates and funding were made available, progressing at least one vaccine through to end of phase 2a for each of the 11 epidemic infectious diseases would cost a minimum of $2.8–3.7 billion ($1.2 billion–$8.4 billion range).
Finally, at least one candidate would progress through to end of phase 2a, out of initial investments of $84–112 million ($23 million–$295 million range) in four to five phase 2 candidates.
A paper from 2017 named The complexity and cost of vaccine manufacturing – An overview took a different route and instead of showing the costs per development phase, uses a financial approach, drilling down the total costs according to its type:
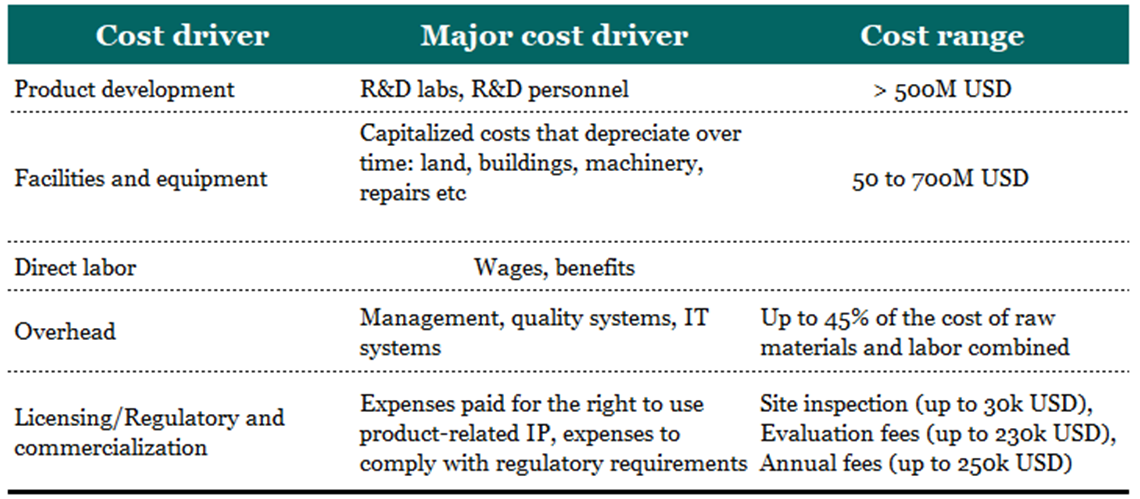
Who pays for the high cost of vaccine development
Governments have the power to make a major difference when it comes to improving public health through creating vaccines. With their backing, companies can develop life-saving treatments that may not be accessible without financial support - plus give extra incentives like production and distribution discounts so more people can get much needed protection against diseases. For example, some countries may offer tax breaks or subsidies on certain vaccines in order to make them more affordable for consumers.
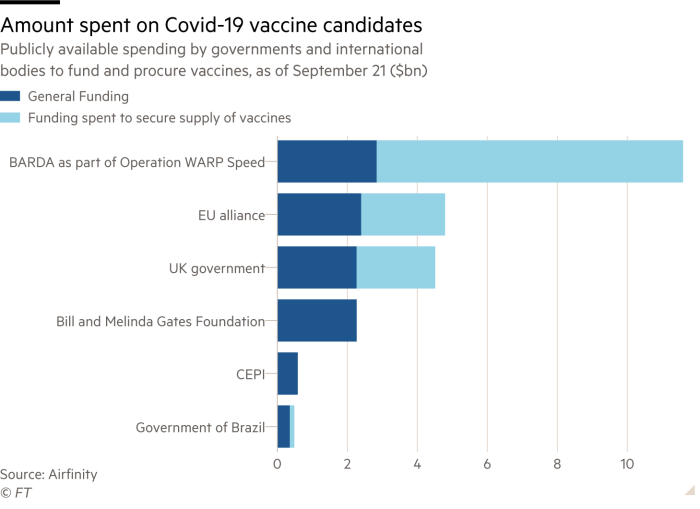
Finally, governments are also working with organizations such as the World Health Organization (WHO) and Gavi, The Vaccine Alliance to support global immunization efforts. These initiatives help make sure that safe and effective treatments are made available to people in developing countries who cannot afford them.
Why the high cost is worth it
According to the World Health Organization, we now have vaccines to prev ent more than 20 life-threatening diseases, helping people of all ages live longer, healthier lives. Immunization currently prevents 3.5-5 million deaths every year from diseases like diphtheria, tetanus, pertussis, influenza and measles.
Vaccines are cost-effective when compared to other forms of healthcare; they’re also much less expensive than treating diseases after they occur. For example, if one person contracts measles or another preventable disease, it can cost thousands of dollars for medical care and lost days of work due to illness—not to mention any long-term effects the disease may have on their health. However, that same person would only need to spend a fraction of the cost on preventive measures such as vaccinations in order to avoid this expense altogether.
Advances in technology are helping to reduce the overall cost of creating vaccines. For example, new manufacturing techniques and processes have made it possible to produce more effective and cheaper vaccines at a faster rate. Additionally, advances in genetic engineering have allowed scientists to create more targeted and specific vaccines that require fewer dosages or boosters. This means that larger numbers of people can be vaccinated against a certain disease at once, which helps to reduce overall costs. In addition, automation and robotics have greatly improved the efficiency of the vaccine production process. This has allowed manufacturers to produce more vaccines with fewer resources, further helping to lower costs.
Conclusion
The cost of making a vaccine can depend on a variety of factors, including the complexity and length of the production process. Advances in technology are helping to reduce the overall cost of creating vaccines, while government subsidies and grants also help to make them more affordable and accessible.
To make sure that everyone has access to safe and effective treatments, it is important that costs associated with the development and distribution of vaccines remain low. This can be achieved by implementing measures such as technological advances, government subsidies and grants, and initiatives to increase accessibility for all consumers.





